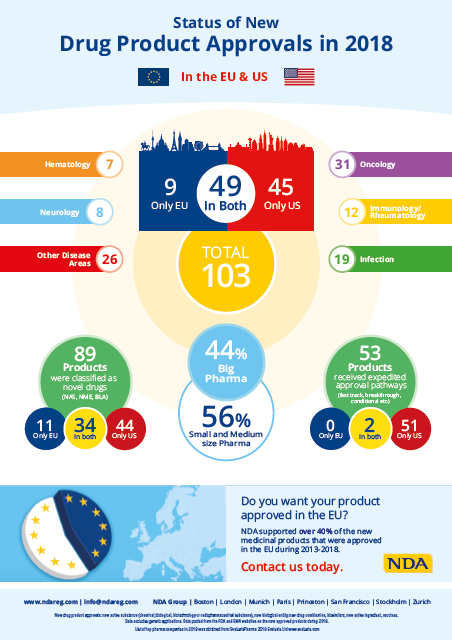
2018 was another exceptional year for the life science industry with a total of 103 new therapeutic drugs (NTDs) approved in EU and US.
Every year NDA reviews the NTD approvals in EU and US from previous year to spot trends and assess the year that has passed. The data is taken from the FDA and EMA websites on the new approved products during 2018 (i). In this review we include NTD product approvals with new active substance (chemical, biological, biotechnology or radiopharmaceutical substance), new biological entity, new drug combinations, biosimilars, new active ingredients and vaccines, but excluded generic and duplicate applications.
The following summary provides an overview of the key findings and an analysis of what the data means for the industry. The data is visually represented in an infographic below.
This year the regulators have ruled positively on some high-profile and high-stakes project. Important new drugs for indications with unmet medical need, for neglected diseases or where exciting new technologies are explored have been approved within the area of neurology (Aimovig, Emgality, Ajovy), infectious disease (Xofluza, Trogarzo), and women health (Orilissa). Important orphan drugs were also approved within neurology (Namuscla, Epidiolex, Onpattro, Tegsedi) and hematology (Crysvita) and advancements within precision medicine have been achieved within oncology (Vitrakvi).
It’s interesting to note that the number of NTD approvals in the two markets has not changed markedly from 2017 to 2018. However, the landscape of approvals in the different markets, indications, and company features has moved somewhat over the last 12 months. Out of the 103 approvals 45 were solely FDA-approved and 9 approved only in EU, indicating an increase in dual market approvals compared to 2017.
There was a rise in the number of NTDs approved for both the US and EU markets from 36 in 2017 to 49 in 2018. This indicates that the joint application strategy was more popular than previous years.
Approvals for oncology and infectious disease products increased in 2018 whilst the number of approvals within hematology, neurology and immunology/rheumatology has decreased during the same time frame.
SUCCESSFUL EXPLORATION OF NOVEL DRUGS
2018 was a significant year for approvals of novel drugs, i.e. treatments based on new active substances. Out of the 103 new drugs, 89 were based on new active substances. This number has increased since the year before when 56 approvals were for novel drugs. This high number of green lights from the agencies follows a few successful years for drug developers. The agencies involvement and support during drug development has increased which also contributes to improvements to strategy rather than to only secure compliance with existing regulations.
After decades of work on migraine prevention drugs finally an antibody-based approach has been approved. Aimovig (Amgen and Novartis) was first approved, and short thereafter came Emgality (Eli Lilly) and Ajovy (Teva). These are self-injected molecules and they all belong to a new class of drugs called calcitonin gene-related peptide receptor (CGRP-R) antagonists. They offer patients treatments that can reduce the number of days with migraine.
Other standouts include new drugs to treat infectious diseases. Xofluza (Roche), a polymerase acidic endonuclease inhibitor, is the first novel flu drug to reach the market in 20 years. This antiviral flu drug is the first that inhibit virus replication. Trogarzo (TaiMed) is a first in class antiretroviral monoclonal antibody approved for the treatment of HIV-1 infection in patients who are multidrug resistant to available treatments. Trogarzo may be able to improve patients’ outcomes when other options have run out.
Women health is historically a neglected field and has been a highly underserved market. However the field has received more attention in recent years. This year, the first new pill, Orilissa (Abbvie) for treatment of moderate to severe pain associated with endometriosis was approved. Orilissa lowers the amounts of estrogens which are expected to decrease the moderate and severe symptoms of endometriosis. It was more than 10 years since the last treatment for endometriosis was approved and there is still a lack of treatment options for this potentially debilitating condition.
A NOBEL PRIZE AND ITS RESULT IN A NOVEL DRUG
One of the highlights of the year was the approval of the first drug that acts by RNA interference (RNAi), Onpattro (Alnylam). The research that lead to the 2006 Nobel Prize in Physiology or Medicine on RNAi was published in 1998 (ii) and has now, 20 years later, successfully been translated into a novel therapy for treatment of a neurology disorder. The transfer of RNAi technology into drug development has been a scientific triumph with great potential to generate treatments for many more indications in the future. Onpattro treat nerve damage caused by hereditary transthyretin (hATTR) amyloidosis and was designated an ‘orphan medicine’.
Last year Tegsedi (Akcea and Ionis), also an antisense oligonucleotide therapy developed for the same disorder, similarly won approvals by FDA and EMA. And more will come, at least six other RNAi therapeutics are in phase III clinical trials for other indications (iii).
SMES AND APPROVED ORPHAN DRUG DESIGNATIONS
In 2018, small and medium sized enterprises (SMEs) contributed with 56% of the approved NTD. We commented on the trend that more and more SMEs are able to take their products through to regulatory approval by themselves last year, and 2018’s figures only strengthen this trend (iv). One driver for this development is the great expansion of the orphan field that provides opportunities to run much smaller late stage trials, thereby limiting the cost of development in a way that suits SMEs. The orphan market accelerated significantly during this year as compared to the previous year.
In 2018 the number of approved new drugs designated orphan status almost doubled in both EU and US, as compared to 2017. Twenty and 42 new orphan drugs were approved in EU and US, respectively, during 2018 (in 2017 12 in EU and 24 in US). Interestingly, nearly 70% of all approved orphan drugs were sponsored by SMEs. This marks great progress of options for patients living with rare diseases, and proves that the drug development companies and the agencies have continued to speed up promising drugs to markets even if the patient groups are limited.
Some of the outstanding contributions to significantly benefit patients living with rare diseases include Epidiolex (GW Research) which is approved by FDA for seizures associated with two rare and severe forms of epilepsy, Lennox-Gastaut syndrome and Dravet syndrome. Its attention is also due to that it is the first FDA-approved drug that contains a purified drug substance derived from Cannabis sativa plant (marijuana). Another interesting new therapy is Namuscla (Lupin).
In EU, Namuscla is the first approved treatment for symptomatic treatment of myotonia in adult patients with non-dystrophic myotonic disorders, a group of inherited muscle disorders where muscles are slow to relax after contraction. These disorders are chronic life-long debilitating conditions characterized by long lasting pain.
Crysvita (Ultragenyx) is an additional exciting new treatment approved in US for patients with X-linked hypophosphatemia (XLH), a rare, inherited form of rickets. Crysvita is the first and only therapy that addresses the underlying cause of X-linked hypophosphatemia.
IMPROVEMENTS WITHIN PRECISION MEDICINE
The ideas of precision medicine are not new, but recent advances in science and technology have helped speed up the pace of this area of research, and major efforts are being invested in the fields.
A notable new oncology drug is Vitrakvi (Loxo and Bayer), a kinase inhibitor for solid tumors in various sites of the body. Vitrakvi became the second cancer therapy to be approved by FDA treating adult and pediatric patients whose cancers have a specific genetic feature, rather than a specific location of the tumor. This approval is a continuation of the new paradigm in the development of cancer drugs that are “tissue agnostic” set by Merck’s Keytruda in 2017.
EXPEDITED APPROVAL OF NOVEL DRUGS
It is obvious that the agencies are working hard to increase the patient access of important medicines where there is huge unmet medical need. In US as many as 53 NTDs were approved through fast track, breakthrough, accelerated approval or priority review approval. In EU only two expedited approvals of NTDs were granted by conditional approval last year.
The trend from 2017 remains during 2018 with more expedited approvals in US than in EU. This might be because of the eligibility to use the expedited pathways is much more limiting in the EU than in the US or that the alternatives in EU for expedited approvals are not as well established with the industry as they are in the US.
NDA SUPPORTED OVER 40% OF THE APPROVALS IN THE EU
NDA had a strong presence in the EU regulatory arena and supported over 40% of the new products approved from 2013 to 2018.
To read the statistics of new drug product approvals from last year click here.
References
i. The data was gathered from the EMA and FDA official websites, as reported on the FDA and the EMA official websites on January 2019.
ii. Fire A., et al. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature 1998; 391:806-811.
iii. Mullard A. FDA approves landmark RNAi drug, Nature Reviews Drug Discovery 2018;17:613
iv. https://www.ndareg.com/europe-vs-usa-new-drug-product-approvals-in-2017/
